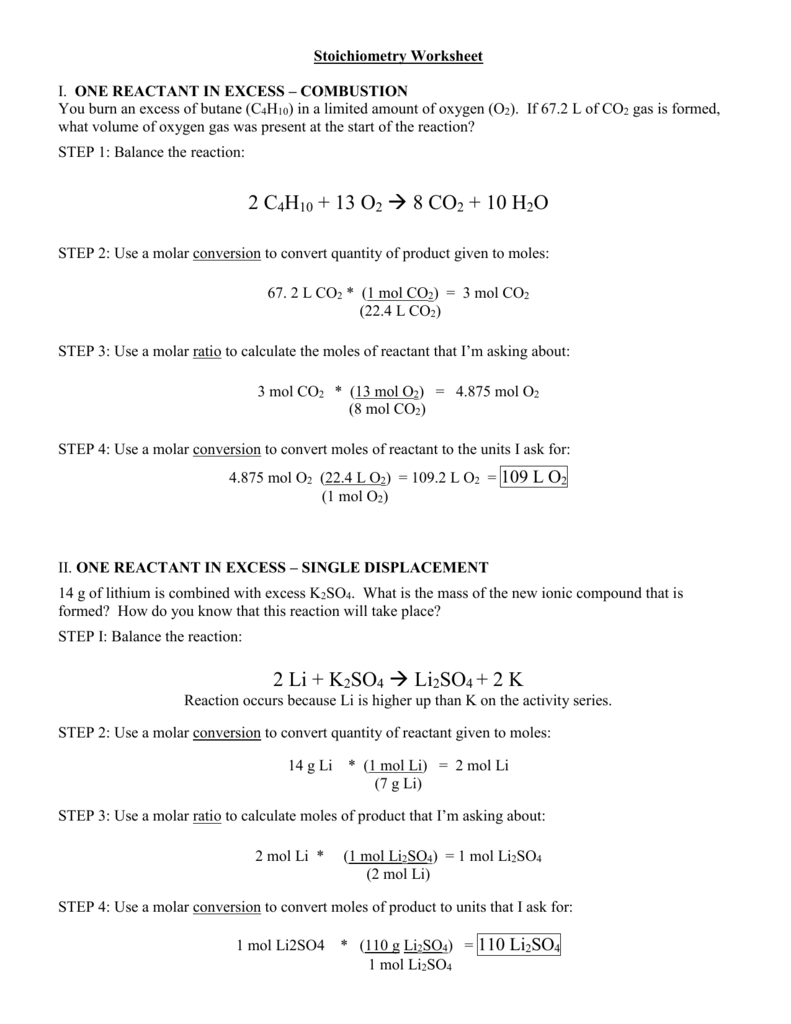
Solution Stoichiometry Worksheet Doc

How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid.
Solution stoichiometry worksheet doc. How many grams of silver chromate will. How much sodium hydroxide can be neutralized by the hydrochloric acid that remains after 38 75 grams of aluminum react with 325 0 grams of. Answers to stoichiometry worksheet. Convert the following number of moles of chemical into its corresponding mass in grams.
0 031 moles of aluminum iodide. 0 436 moles of ammonium chloride. 500 m silver nitrate are added. Solve the following stoichiometry grams grams problems.
Stoichiometry worksheet key solutions doc. 2 agno3 aq k2cro4 aq ag2cro4 s 2 kno3 aq 2. Determine the excess reactant and the grams of it that remains the moles of precipitate that form and the grams of the other product formed. Ml of 0 500 m silver nitrate are added to 100.
2 360 moles of lead ii oxide. How many grams of silver chromate will precipitate when 150. 4ag s 2h2s g o2 g 2ag2s s 2h2o l m 1 23 mg m. 2 naoh h2so4 2 h2o na2so4.
400 m potassium chromate. Worksheet for basic stoichiometry. 2 using the following equation. 0 50 moles of calcium nitrate.
How many grams of silver chromate will precipitate when 150. 1 using the following equation. Worksheet 9 3 1 stoichiometry in solutions 1. 2 agno 3 aq k 2 cro 4 aq ag 2 cro 4 s 2 kno 3 aq 0 150 l agno 3 0 500 moles agno 3 1 moles ag 2 cro 4 331 74 g ag 2 cro 4.
1 how many grams of calcium phosphate can be produced from the reaction of 2 50 l of 0 250 m calcium chloride with and excess of phosphoric acid. Solve the following solutions stoichiometry problems. Ml of 0 400 m potassium chromate. How many ml of 0.
Solution stoichiometry worksheet solve the following solutions stoichiometry problems. 1 077 moles of magnesium phosphate. What mass of silver sulfide can be made from 1 23 mg of hydrogen sulfide h2s obtained from a rotten egg. View wkst 9 3 1 problems stoichiometry in solutions doc from chem misc at university of waterloo.
Mole mass conversions. This solution reacts with 35 0 grams of silver nitrate.