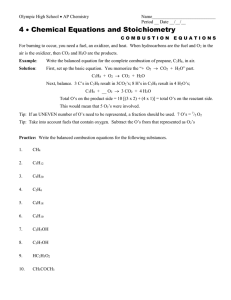
Enthalpy Stoichiometry Worksheet Doc

The balanced chemical equation provided equivalences that we used to construct conversion factors for example in the balanced chemical equation.
Enthalpy stoichiometry worksheet doc. Enthalpy stoichiometry worksheet period how much heat will be released when 6 44 g of sulfur reacts with excess 02 according to the following equation. Thus ah for the reaction 890 4 kj. 840 g mg 4400 g hno3 14a. 2s 3o 2 2so 3 h 792 kj the h value given in the equation is the amount of heat transferred when 2 moles of sulfur and 3 moles of oxygen react.
In chapter 5 stoichiometry and the mole we related quantities of one substance to another in a chemical equation by performing calculations that used the balanced chemical equation. Stoichiometric calculations and enthalpy changes. O 2 co 2 c. 2 h 2 g o 2 g 2 h 2 o ℓ.
In chapter 5 stoichiometry and the mole we related quantities of one substance to another in a chemical equation by performing calculations that used the balanced chemical equation. Enthalpy stoichiometry name chem worksheet 16 3 example how much heat is produced when 85 g of sulfur reacts according to the reaction below. Given that the heat of formation of potassium chloride is 436 kj mol and the heat of formation of potassium chlorate is 391 kj mol determine the heat of reaction. Given the following equation.
C 02 vt ah 393 5 kj. Enthalpy stoichiometry name chem worksheet 16 3 example how much heat is produced when 85 g of sulfur reacts according to the reaction below. O 2 h 2o d. 2 4 x 104 kj 12.
What quantity of heat is produced if 32 g of cyclohexane c6h12 9 o2 6 co2 6 h2o 3 690 kj. The combustion of methane chs releases mole of methane is burned 890 4 k are given off to the surroundings. Since the reaction of 1 mol of methane released 890 4 kj the reaction of 2 mol of methane would release. That is when one 1.
C 4h 10 o 2 b. Refer again to the combustion reaction of methane. 2 kclo 3 2 kcl 3 o 2 a. 2 h 2 g o 2 g 2 h 2 o ℓ.
2 c 4h 10 13 o 2 8 co 2 10 h 2o show what the following molar ratios should be. 2s 3o 2 2so 3 h 792 kj the h value given in the equation is the amount of heat transferred when 2 moles of sulfur and 3 moles of oxygen react. 18 0 mol o 2 3. C 4h 10 co 2 e.
How many moles of o 2 can be produced by letting 12 00 moles of kclo 3 react. Stoichiometry worksheet 1 answers 1. Enthalpy stoichiometry name chem worksheet 16 3. Enthalpy worksheet enthalpy worksheet 890 4 kj mol of heat.
This means that the products have 890 4 kj less energy stored in the bonds than the reactants. C 4h 10 h 2o 2. 1 75 mol cu 11a. 27 8 g fe 13a.
Given the following equation. The balanced chemical equation provided equivalences that we used to construct conversion factors for example in the balanced chemical equation. Chemistry problems that involve enthalpy changes can be solved by techniques similar to stoichiometry problems.