Electron Configuration Of Potassium In Ground State

Has approximately the same energy.
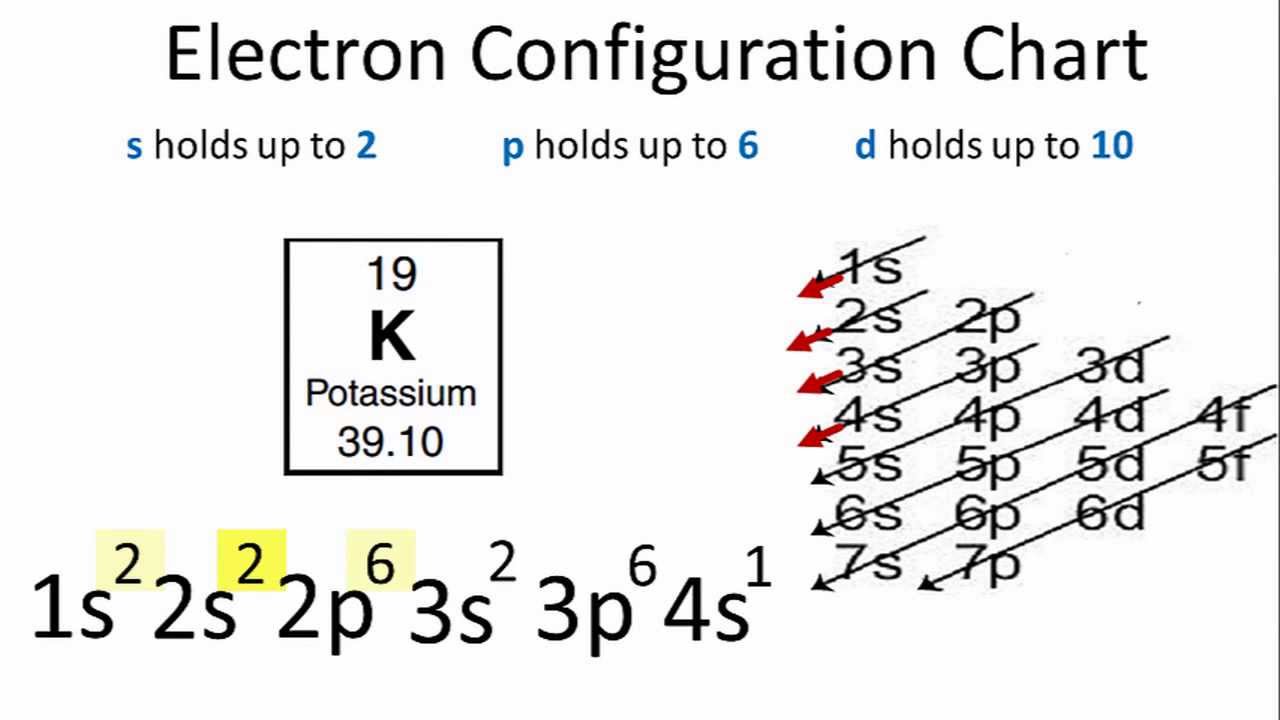
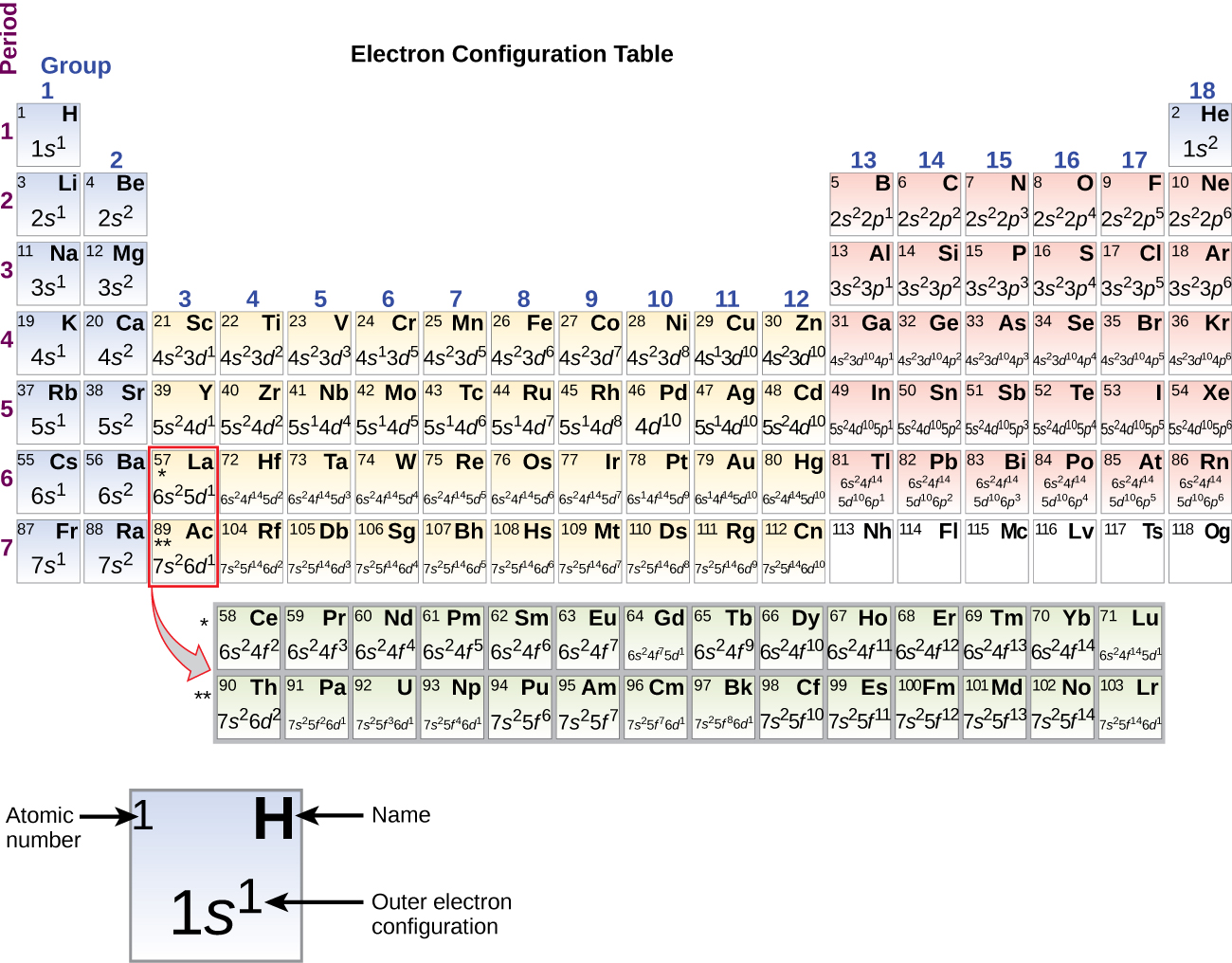
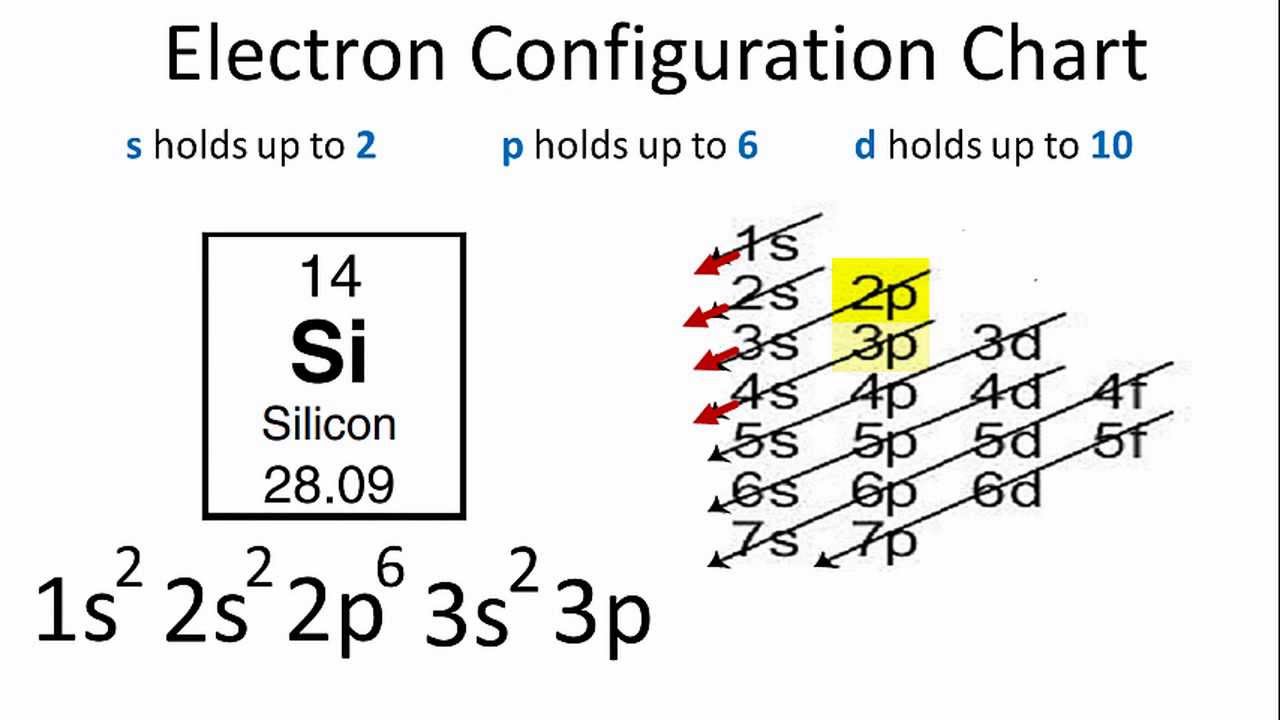
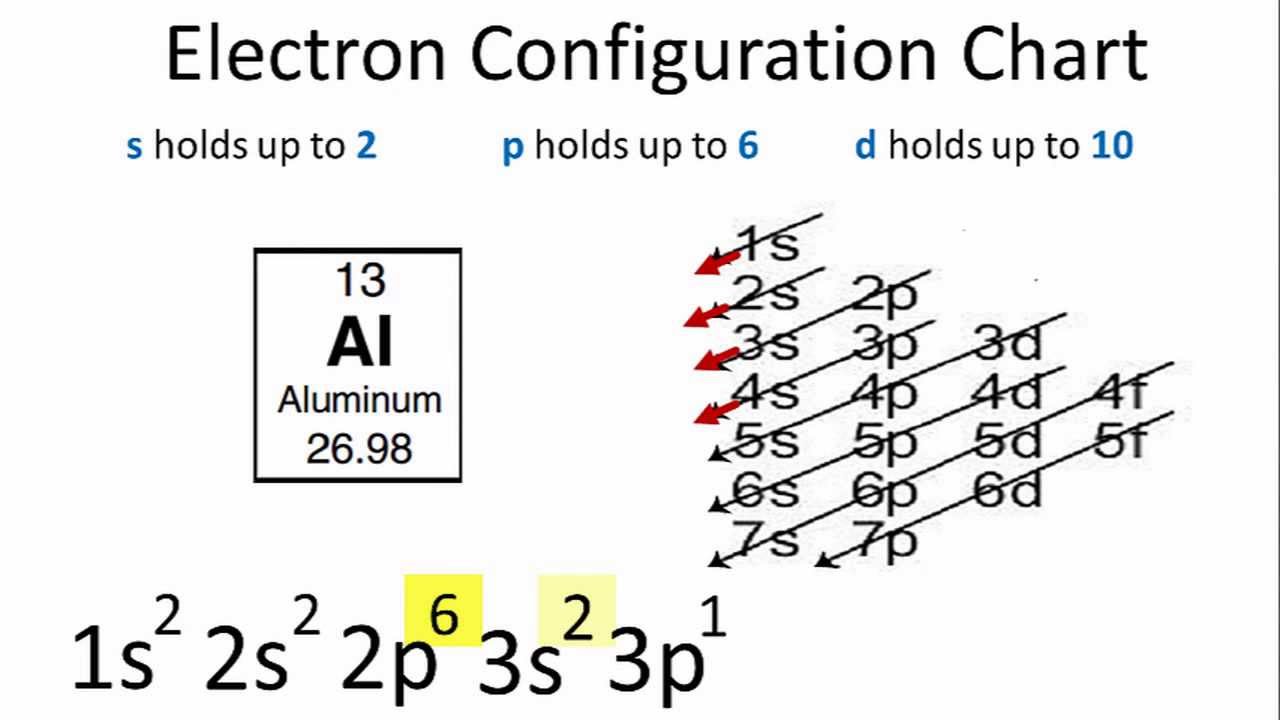
Electron configuration of potassium in ground state. Electron configuration and oxidation states of potassium. The p orbital can hold up to six electrons. For the ground state electron configuration then we get since there is only one possible ground state electron configuration for a neutral element any other arrangement of potassium s 19. Possible oxidation states are 1.
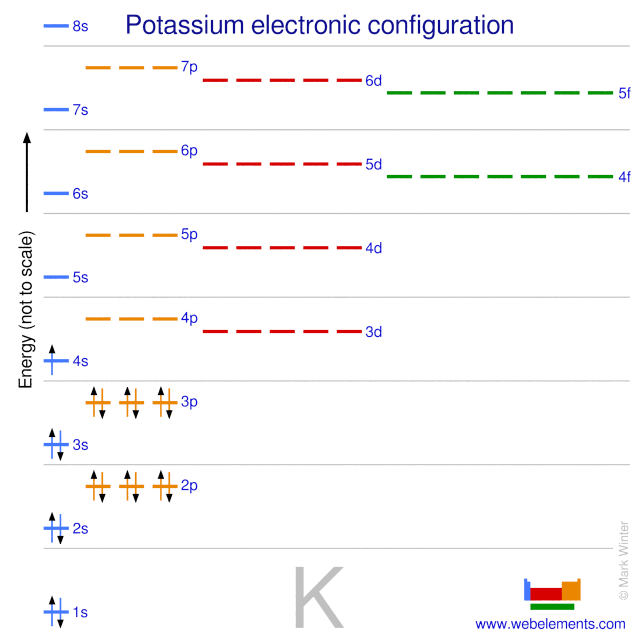
Potassium atoms have 19 electrons and the shell structure is 2 8 8 1. 1s 2 2s 2 2p 6 3s 2 3p 6 4s this tells you that compared to the 3d level for this atom the 4s level answer. The noble gas shorthand configuration is ar 4s1. 4s 1 and the term symbol is 2 s 1 2.
The following ground state electron configuration of potassium shows that for the ground state of the potassium atom the outermost electron is in a 4s state. In writing the electron configuration for potassium the first two electrons will go in the 1s orbital. We ll also look at why potassium forms a 1 ion and how the electron config. Potassium is a chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure.
Has values greater than or equal to 1. Since 1s can only hold two electrons the next 2 electrons for potassium go in the 2s orbital. Has three electrons per orbital each with identical spins. The chemical symbol for potassium is k.
The next six electrons will go in the 2p orbital. Hund s rule states that the most stable arrangement of electrons for a ground state electron configuration has a filled valence shell of electrons. The electron configuration for a ground state potassium atom is 1s22s22p63s23p64s1. Electron configuration of potassium is ar 4s1.
Has the maximum number of unpaired electrons all with the same spin. The ground state electron configuration of ground state gaseous neutral potassium is ar.