Electron Configuration Of Potassium Cation
The next electron is added to complete the 4 s subshell and calcium has an electron configuration of ar 4 s2.
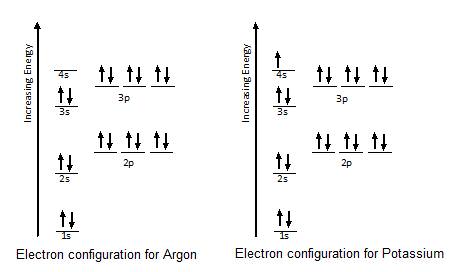
Electron configuration of potassium cation. What is the electron configuration of the cation k. 2014 wayne breslyn method 2. Since 1s can only hold two electrons the next 2 electrons for potassium go in the 2s orbital. If the electron configuration of a ground state potassium atom is 1s 228 22p 63s 1.
Hence the electron configuration of potassium k is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 potassium ion is formed by losing an electron. None of the above. 1s2 2s2 2p6 3s2 3p6 4s2 3. The electron configuration of the potassium cation k would be 1s22s22p6 15225220635 1512s22p6351 1s22s22p6352 none of the above.
Iodized salt is table salt nacl supplemented with potassium iodide which is made of the ions k and i. In writing the electron configuration for potassium the first two electrons will go in the 1s orbital. We ll also look at why potassium forms a 1 ion and how the electron config. Using the electron config.
If the electron configuration of a ground state potassium atom is 1s22s22p63s1 the electron configuration of the potassium cation k would be. What is the formula of the ion formed when potassium achieves a noble gas configuration a. The next six electrons will go in the 2p orbital. 1s22s22p6 choose the answer that best completes the following statement.
The p orbital can hold up to six electrons. As far as the number of neutrons generally the number of protons neutrons will add up to equal the atomic mass. Hence potassium corresponds to li and na in its valence shell configuration. In this video we will write the electron configuration for k the potassium ion.
When an aluminum atom reacts so as to attain a noble gas electron configuration. Conceptest dealing with electron configurations of ions. 1s2 2s2 2p6 3s2 3p6 4s1 2.