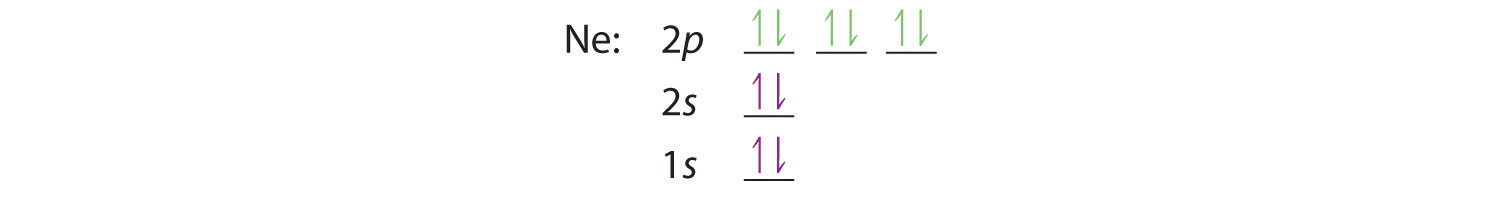Electron Configuration Of Neon Meaning
To save room the configurations are in noble gas shorthand.
Electron configuration of neon meaning. For example we know that when it creates an ion oxygen often forms2 ions. In its usual configuration this would add 2 electrons to make the new configuration. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals.
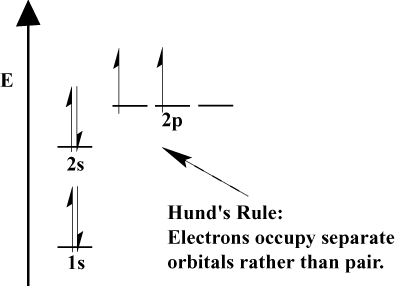
H 1s 1 he 1s 2 li 1s 2 2s 1 be 1s 2 2s 2 b 1s 2 2s 2 2p 1 c 1s 2 2s 2 2p 2 n 1s 2 2s 2 2p 3 o 1s 2 2s 2 2p 4 f 1s 2 2s 2 2p 5. Notice that for neon as for helium all the orbitals through the 2 p level are completely filled. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. For example the electron configuration of a neon atom is 1s 2 2s 2 2p 6.
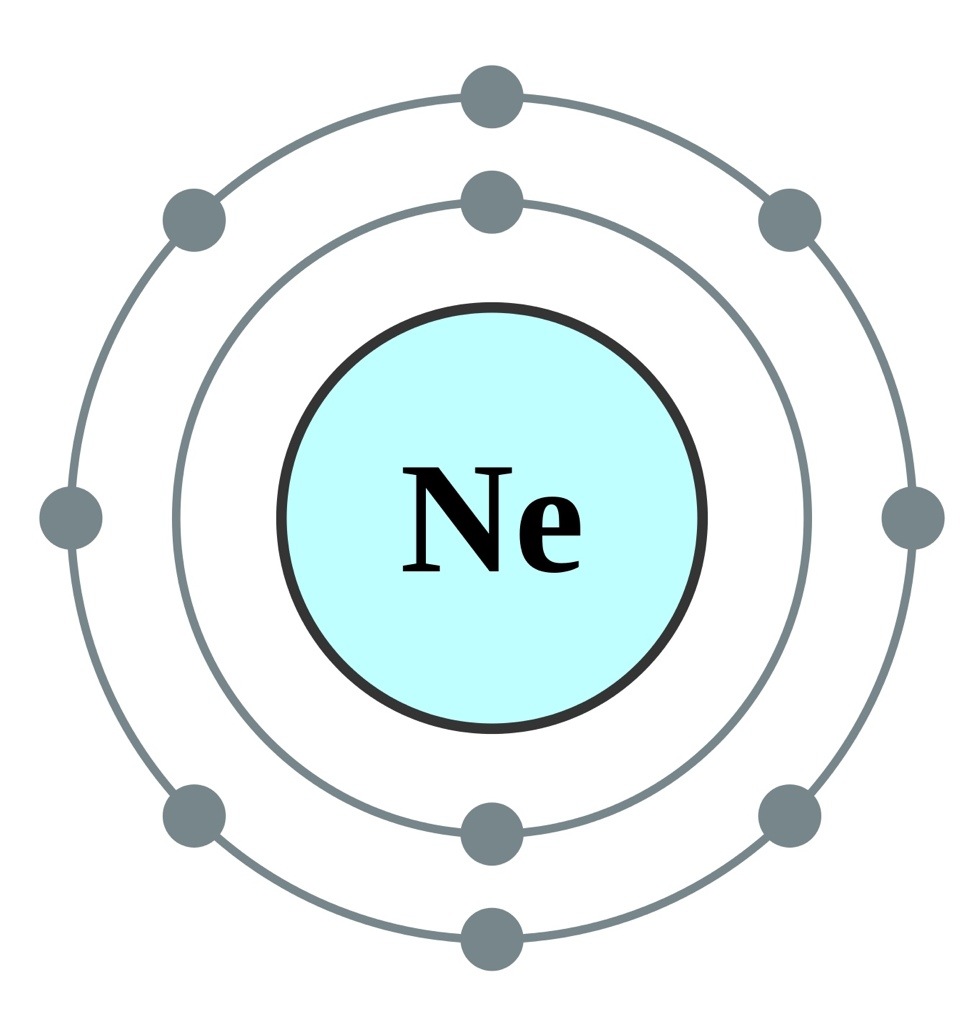
For example sodium has one 3s electron in excess of the noble gas neon chemical symbol ne atomic number 10 and so its shorthand notation is ne 3s 1. The remaining six electrons will go in the 2p orbital. The k and l shells are shown for a neon atom. Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital.
Electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. You must remember with 10 electrons that the electron structure of oxygen is now exactly the same as that of neon. Therefore the ne electron configuration will be 1s 2 2s 2 2p 6. For example the electron notation of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3 while the noble gas notation is ne 3s 2 3p 3.
Screened from the nucleus by intervening electrons the outer valence electrons of the atoms of the heavier noble gases are held less firmly and can be removed ionized more easily from the atoms than can the electrons of the lighter noble gases. Neon is the tenth element with a total of 10 electrons. The more detailed version of the electronic configuration reflects the sub shells in the n 1 and n 2 shells where the n 1 shell just has a single s sub shell containing a single s orbital which can hold up to 2 electrons. This list of electron configurations of elements contains all the elements in increasing order of atomic number.
The electron configuration for the first 10 elements. Often a shorthand method is used that lists only those electrons in excess of the noble gas configuration immediately preceding the atom in the periodic table. The n 1 shell can only hold 2 electrons so the remaining 8 electrons fill the n 2 shell. To do this the nearest noble gas that precedes the element in question is written first and then the electron configuration is continued from that point forward.
Neon has atomic number 10 so a neon atom has 10 protons in its nucleus and therefore 10 electrons.