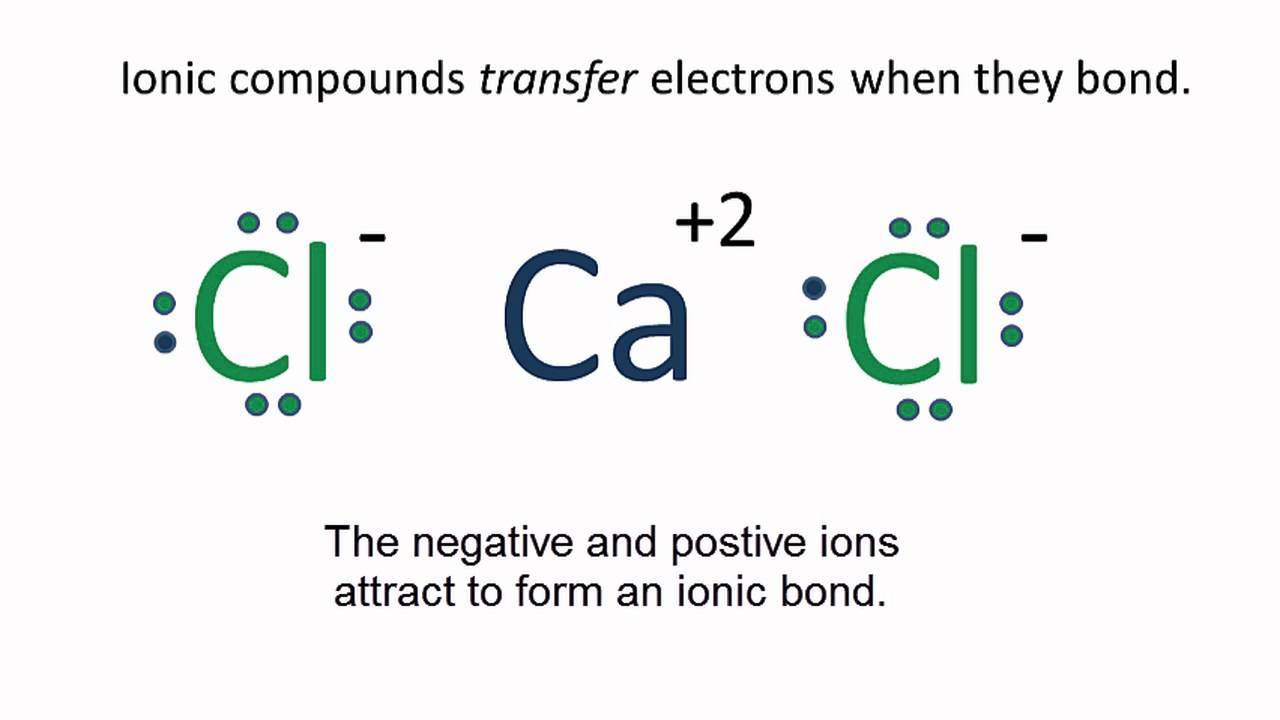Electron Configuration Of Calcium Chloride
Calcium chloride dihydrate for molecular biology 99.
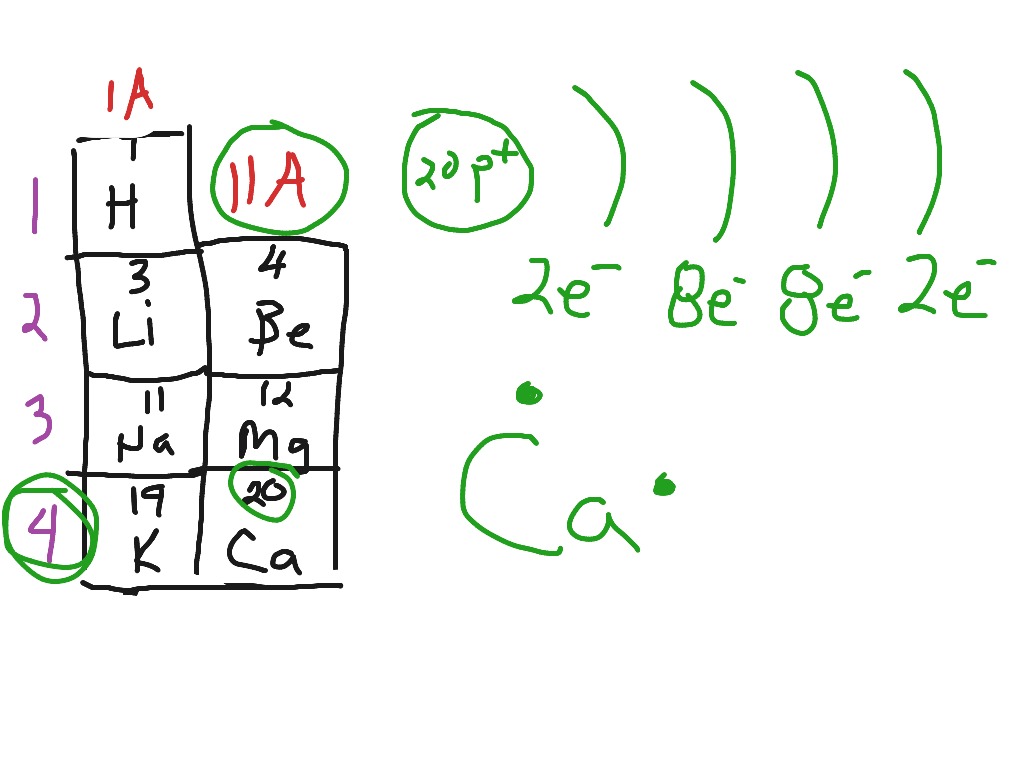
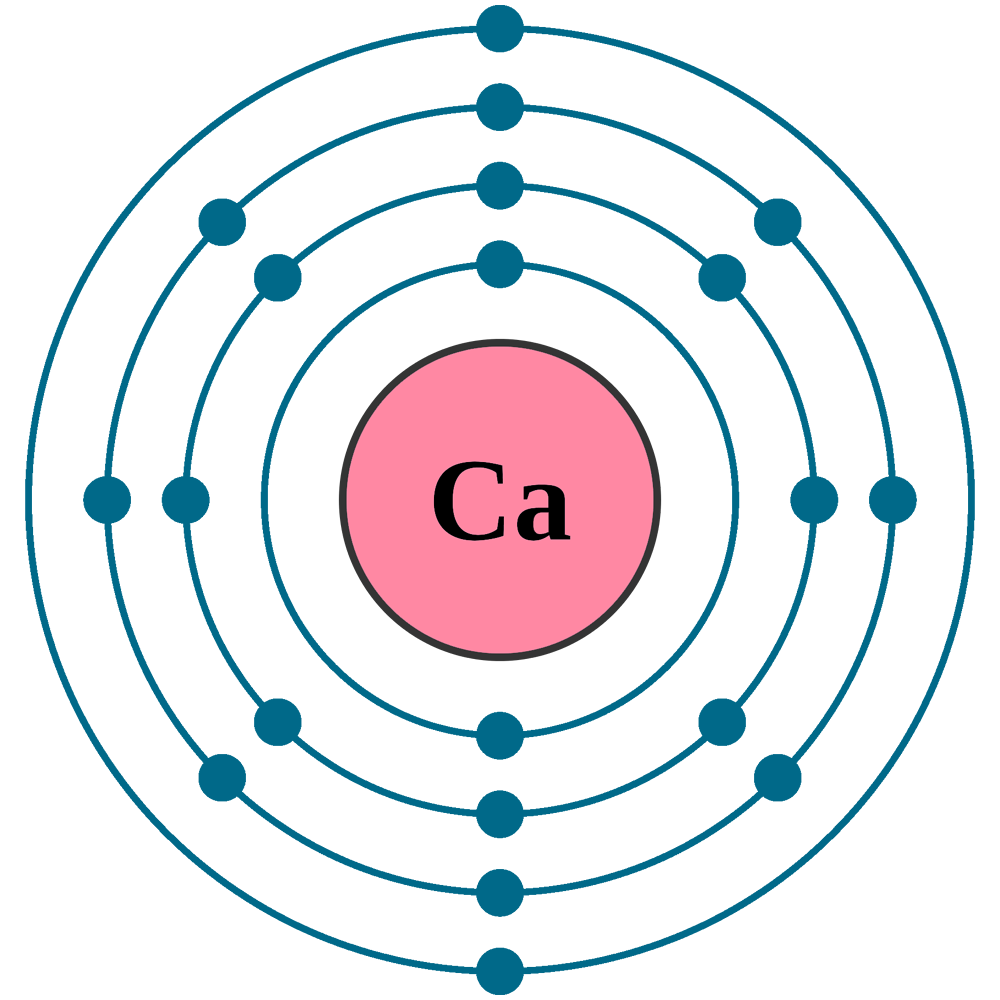
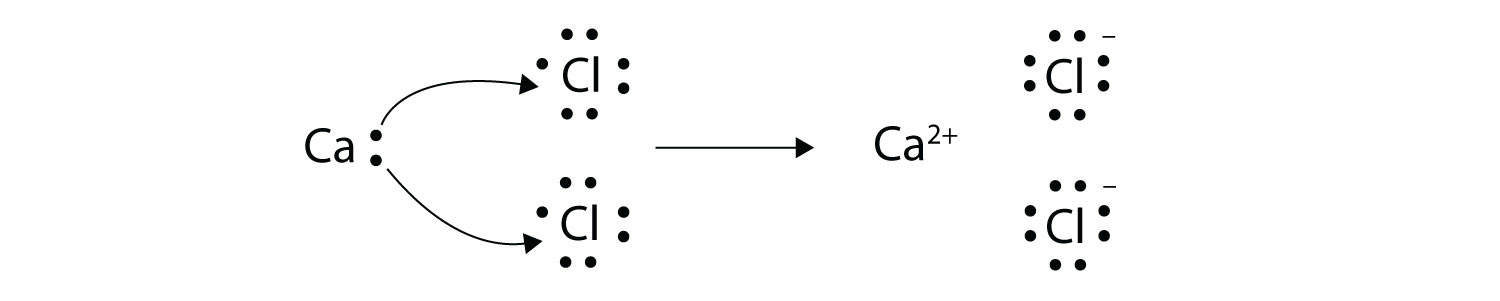
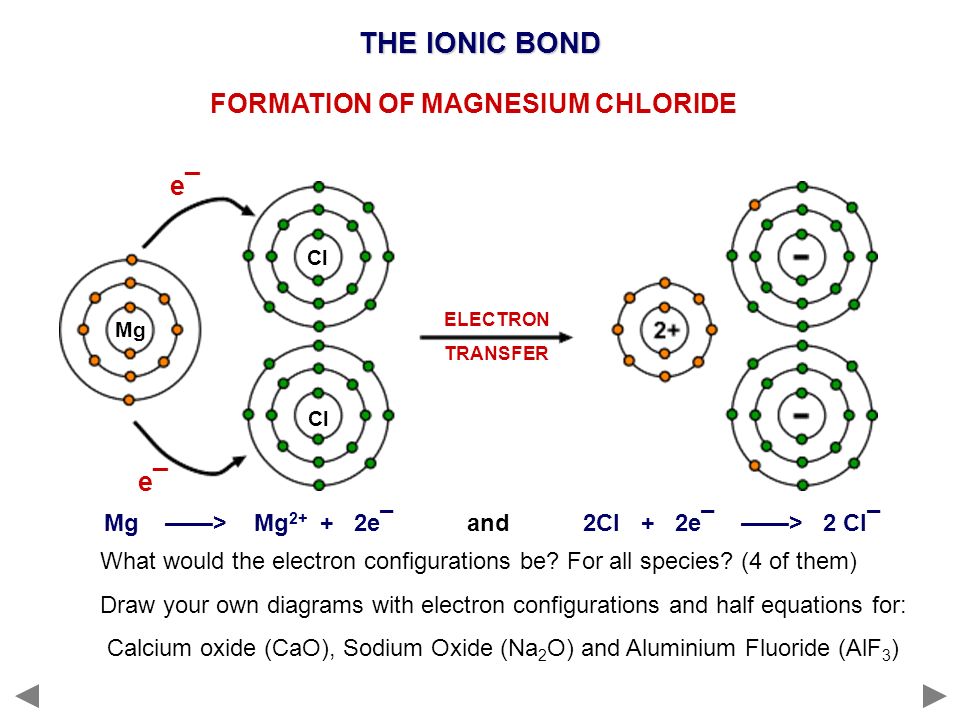
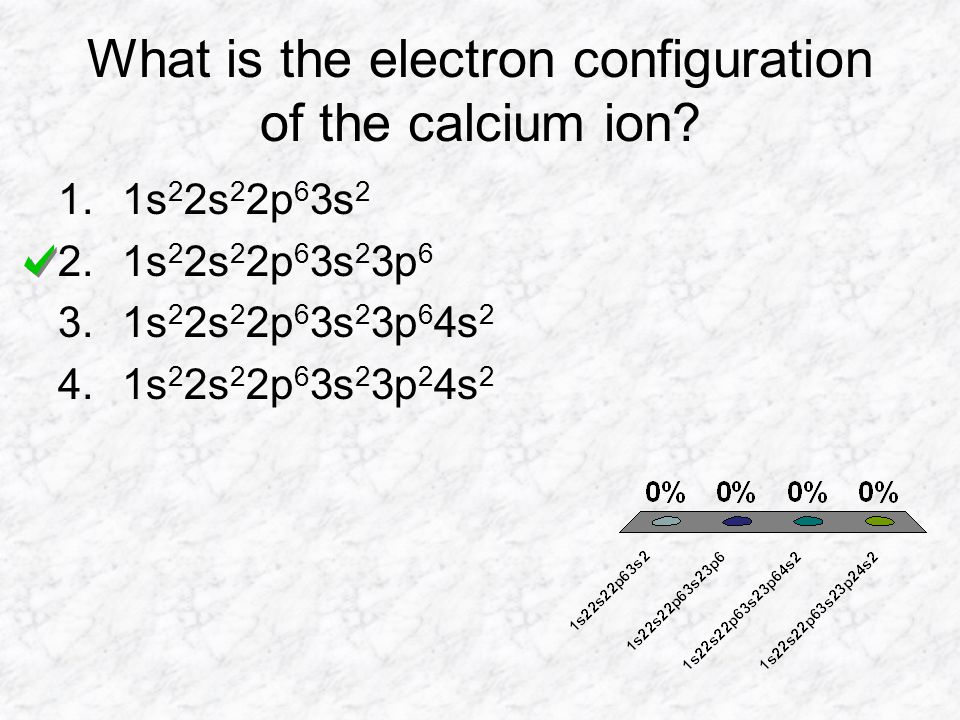
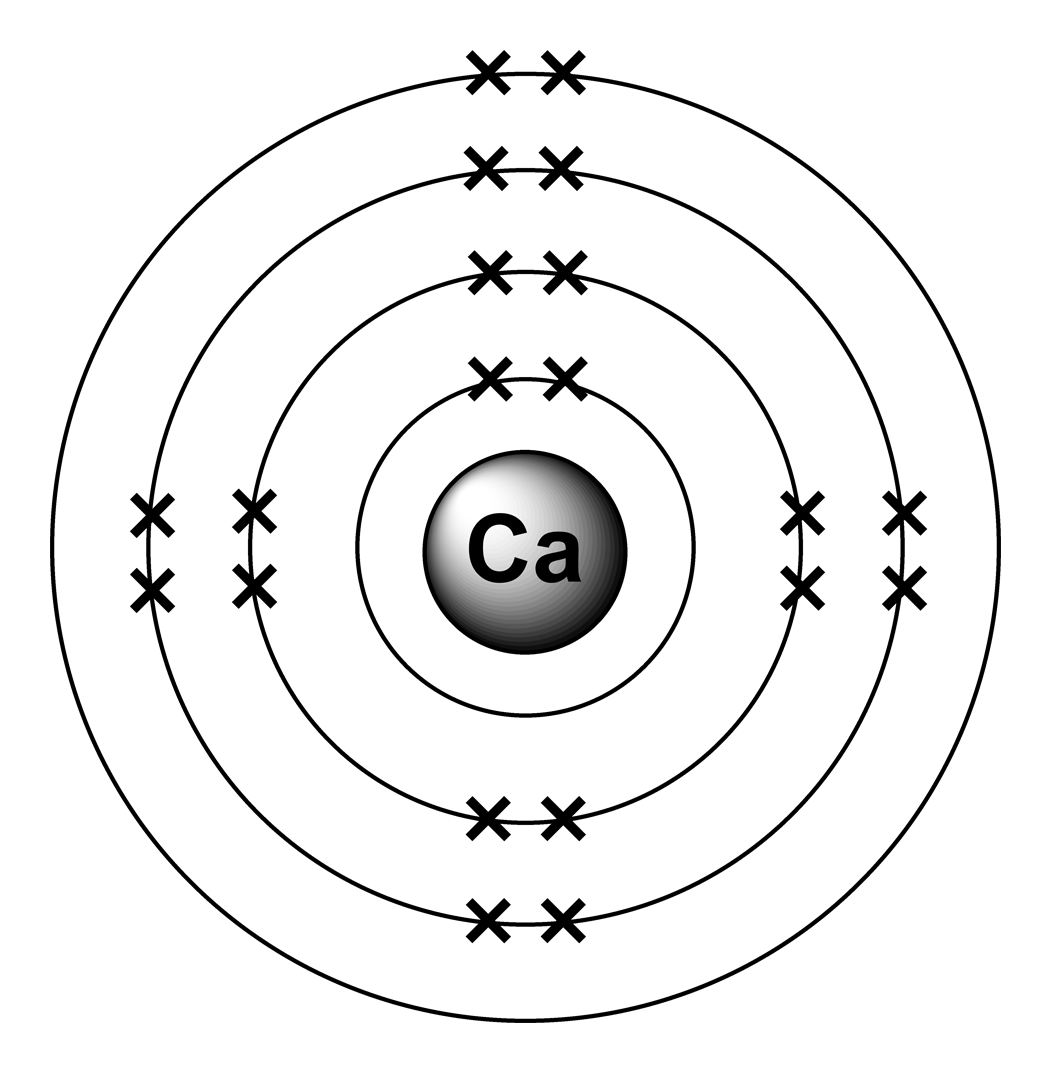
Electron configuration of calcium chloride. The effects of amrinone and calcium chloride on pulmonary vasculature and biventricular function in sheep with acute lung injury were studied. In the answer choices provided choice b is the only electron configuration that contains 18 electrons making b the correct answer. Therefore ca 2 has a total of 18 electrons. Calcium chloride dihydrate jis special grade 99 0 103 0.
Electron configuration elementelectron configuration klinaca1s2 2s2 2p6 3s2 3p6 4s2table 4. Calcium chloride dihydrate reagentplus r 99 0. Calcium chloride dihydrate tested according to ph eur. Determine the volume of a 2 00m calcium chloride solution that would be needed to exactly react with 0 0750l of 1 00m.
Color of light emitted by salt types saltcolorwavelength liclnaclyellow colored flame589nm kclpink colored flame766nm cacl2orange between 591 603nm. Lithium chloride licl sodium chloride nacl potassium chloride kcl calcium chloride cacl2 table 3. Oso 4 1 4 76 32 108 1 2 3 instructions. Calcium chloride dihydrate vetec tm reagent grade 99.
Al 2 o 3 aluminum oxide iii instructions. There is a considerable amount of chloride ions in sea water. Cuso 4 copper sulfate 15. Pci 3 1 3 2.
Sii 4 silicon tetraiodide 19. Chloride also exists naturally in water sources and this is the most common anion in the nature. Ccl 4 1 4 12 144 156 1 0 92 6. The electron configuration of chloride is 1 s2 2 s2 2 p6 3s 2 3p 6.
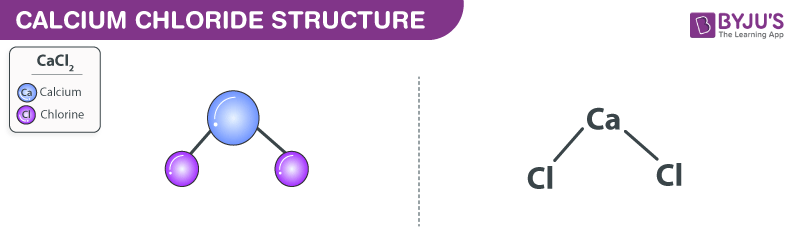
Cacl 2 calcium chloride 14. Pci 5 phosphorus chloride 18. Write out the electron configurations of each of the metals in the salt compounds used in table 3. Seven sheep were ventilated with a tidal volume of 10 12 sq ml of 40 or 5 mm hg 5 3 or 0 7 kpa after acute lung injury was induced with up to 30 mg kg of ethchlorvynol biventricular function and hemodynamic profiles were.
Chloride exists in ionic compounds such as sodium chloride calcium chloride and hcl which are ionic. Sbf 5 1 5 122 95 207. Name the following ionic compounds. Name the following molecular compounds.
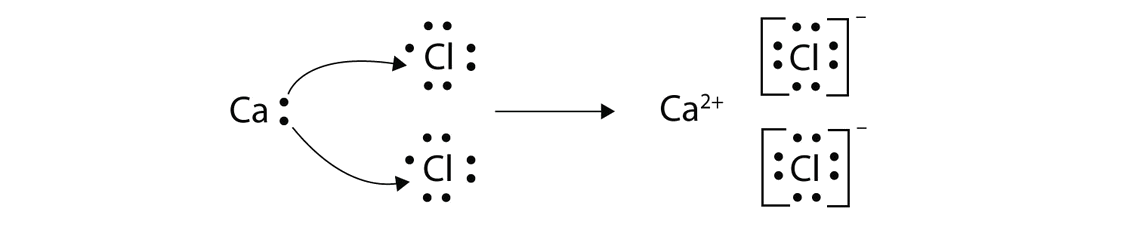
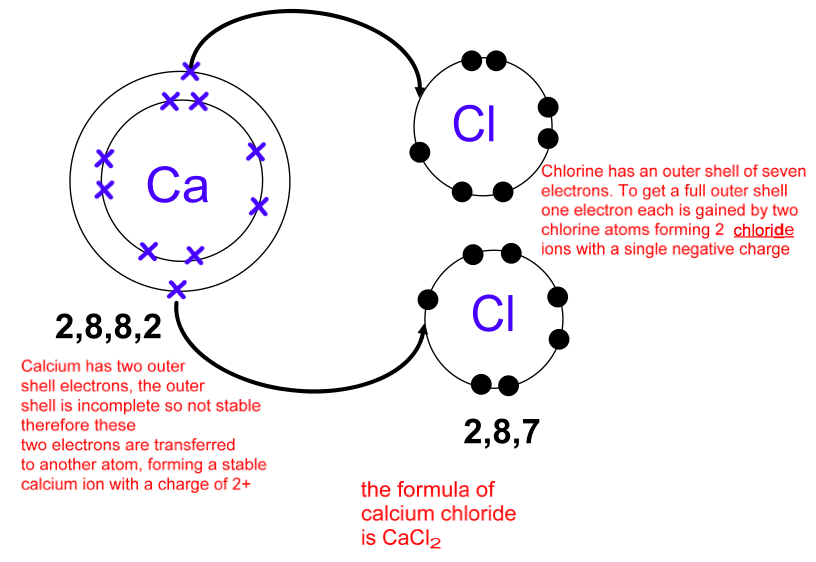
This question asks the examinee to determine the electron configuration of the calcium ion as it exists in calcium chloride. Potassium is already done as an example for you. Bacl 2 1 2 137 72 209 72 209 3 137 209 7 1 1 4 4. Mgcl 2 magnesium chloride 13.
Calcium chloride reacts with sodium carbonate aq to form solid calcium carbonate and aqueous sodium chloride. Calcium in its elemental state has a total of 20 electrons. Write the ground state electron configuration for the chloride ion. Cl 2 o 7 dichlorine heptoxide 17.
The periodic table is very helpful and can be used as guide. Bai 2 1 2 137 254 391 0 4 0 7 1 0 6 5.